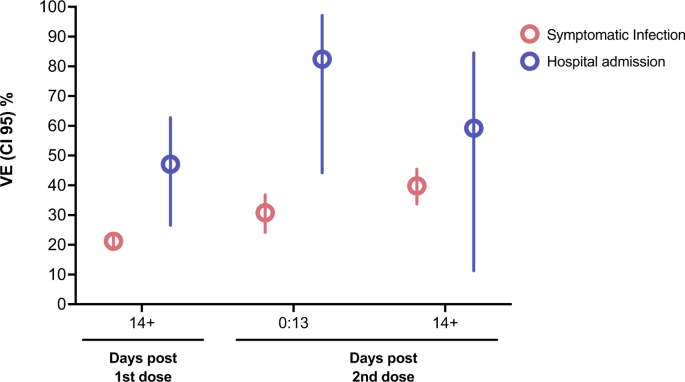
Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the Omicron period

Frenck, R. W. et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N. Engl. J. Med. 385, 239–250 (2021).
Walter, E. B. et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N. Engl. J. Med. 386, 35–46 (2022).
Center for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). (2020). Available at: https://emergency.cdc.gov/han/2020/han00432.asp. (Accessed: 14th May 2020).
Stein, M. et al. Project Report The Burden of COVID-19 in children and its prevention by vaccination: a joint statement of the israeli pediatric association and the israeli society for pediatric infectious diseases. Vaccines 10, 81 (2022).
Ministério da Saúde do Brasil. Nota Técnica No6/2022-Secovid/Gab/Secovid/Ms. (2022).
Jara, A. et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in children and adolescents: a large- scale observational study. SSRN (2022).
Jara, A. et al. Effectiveness of CoronaVac in children 3 to 5 years during the omicron SARS-CoV-2 outbreak. Nat. Med. 28, 1377–1380 (2022).
Florentino, P. T. V. et al. Vaccine effectiveness of two-dose BNT162b2 over time against COVID-19 symptomatic infection and severe cases among adolescents: test negative design case control studies in Brazil and Scotland. The Lancet Infectious Diseases. 1–23 (2022).
Andrews, N. et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 386, 1532–1546 (2022).
Buchan, S. A., Nguyen, L., Wilson, S. E., Kitchen, S. A. & Kwong, J. Vaccine effectiveness of BNT162b2 against Omicron and Delta outcomes in adolescents. medRxiv 2022.04.07.22273319 (2022).
Veneti, L. et al. Vaccine effectiveness with BNT162b2 (Comirnaty, Pfizer-BioNTtech) vaccine against reported SARS-CoV-2 delta and omicron infection among adolescents, Norway, August 2021 to January 2022. SSRN Electron. J. 2, 1–11 (2022).
Dorabawila, V. et al. Effectiveness of the BNT162b2 vaccine among children 5–11 and 12–17 years in New York after the emergence of the Omicron Variant. medRxiv 2022.02.25.22271454 (2022).
Sacco, C. et al. Articles Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: a retrospective analysis of January-April, 2022. Lancet 2, 1–7 (2022).
Vandenbroucke, J. & Pearce, N. Test-negative designs differences and commonalities with other case–control studies with “other patient” controls. Epidemiology 30, 838–844 (2019).
Dawood, F. S., Porucznik, C. A., Veguilla, V. & Al, E. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City, New York. Jama Pediatrics 176, 59–67 (2022).
Mensah, A. A. et al. Risk of SARS-CoV-2 reinfections in children: a prospective national surveillance study between January, 2020, and July, 2021, in England. Lancet Child Adolesc. Health 6, 384–392 (2022).
Lipsitch, M., Goldstein, E., Ray, G. T. & Fireman, B. Depletion-of-susceptibles bias in influenza vaccine waning studies: how to ensure robust results. Epidemiol. Infect. 147, e306 (2019).
Katikireddi, S. V. et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet 399, 25–35 (2022).
Cerqueira-Silva, T. et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat. Med. 28, 838–843 (2022).
Cerqueira-Silva, T. et al. Vaccination plus previous infection: protection during the omicron wave in Brazil. Lancet Infect. Dis. 22, 1–2 (2022).
Cerqueira-Silva, T. et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case-control study. Lancet Infect. Dis. 22, 791–801 (2022).
Vandenbroucke, J. P., Brickley, E. B., Vandenbroucke-Grauls, C. M. J. E. & Pearce, N. A test-negative design with additional population controls can be used to rapidly study causes of the SARS-COV-2 epidemic. Epidemiology 31, 836–843 (2020).
Fiocruz. GISAID. Rede Genômica (2020). Available at: http://www.genomahcov.fiocruz.br/#. (Accessed: 15th May 2022)
Allik, M. et al. Small-area Deprivation Measure for Brazil: Data Documentation. University of Glasgow (2020). Available at: https://researchdata.gla.ac.uk/980/. (Accessed: 15th May 2020).
Vienna, A. R. Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; (2021).
Wickham, H. et al. Welcome to the Tidyverse Tidyverse package. J. Open Source Softw. 4, 1–6 (2019).
van Buuren, S. & Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Firth, D. “Bias reduction of maximum likelihood estimates.”. Biometrika 82, 667 (1995).
Benchimol, E. I. et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 12, 1–22 (2015).