The association between Acinetobacter baumannii infections and the COVID-19 pandemic in an intensive care unit | Scientific Reports

Study population, diagnostic criteria, and sample collection
The study was conducted at Koç University Hospital, Istanbul, Turkey. All the patients infected with A. baumannii in the ICU were included, covering 27 months for both the pre-pandemic (January 2018–March 2020) and the pandemic (April 2020–June 2022) era. Clinical findings for sepsis18, pneumonia19, and/or urinary tract infection20 and isolation of A. baumannii from blood, urine, and/or tracheal aspirate were required for the diagnosis of infection. Bacterial identifications were done using MALDI-TOF VITEK® MS (BioMerieux, March L’Etoile, France).
Antibiotic susceptibility testing
Antimicrobial susceptibility testing was performed for piperacillin-tazobactam, ceftazidime, imipenem, meropenem, amikacin, gentamicin, ciprofloxacin, tigecycline, and colistin by using the Vitek2 automated system (BioMerieux, March L’Etoile, France). The MIC breakpoints were evaluated according to the EUCAST criteria21. A. baumannii isolates testing resistant to at least one antibiotic of more than three antibiotic classes were identified as multidrug-resistant (MDR) isolates. The presence of carbapenemase was checked using the RAPIDEC® Carba NP kit (BioMerieux, March L’Etoile, France). Confirmation of amikacin susceptibility was verified using the disk diffusion method with 30 μg of amikacin-containing discs.
Pulsed-field gel electrophoresis and cluster analysis
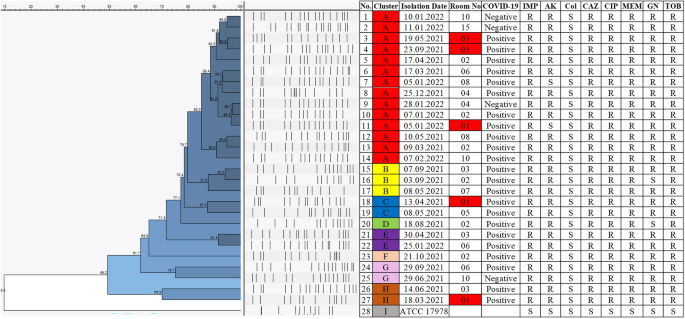
Pulsed-field gel electrophoresis (PFGE) method was done to assess the genetic clonality of 27 MDR A. baumannii isolates collected from the patients admitted to the ICU between January 2021 and February 2022 during the COVID-19 pandemic. The salmonella standard protocol of CDC PulseNet was used with minor modifications22. Bacterial samples cultured overnight were adjusted to the cell concentration of 0.45 absorbance at 590 nm (2.00 McFarland Standard) and suspended in the cell suspension buffer (100 mM Tris (pH 8.0), 10 mM EDTA). Low melting 1% SeaKem agarose was used for the formation of the plugs. Genomic DNA was lysed overnight at 55 °C in a lysis solution (50 mM Tris, 50 mM EDTA, proteinase K (20 mg/ml), pH 8.0). ApaI (50U per isolate) was used as the restriction enzyme and incubation was done at 37 °C for 4 h. Lambda Ladder PFG Marker (NEB, US) was used as the molecular size marker and 1% SeaKem agarose was used for gel preparation. Run conditions were set as, temperature 14 °C; voltage 6 V/cm; switch angle, 120°; switch ramp 7–20 s with the run duration of 18 h on Chef Mapper II (Chef Mapper, Bio-Rad Laboratories, Hercules, CA, USA). Ethidium bromide was used for gel staining and visualization of the results was done using ChemiDoc™ XRS + System with Image Lab™ Software (BIO-RAD, USA).
Acinetobacter baumannii ATCC 17978 was used for the normalization of bands on different gels and the oldest isolate was used as the reference strain, timewise. Results were analyzed using Bionumerics 7.6 software (Applied Maths NV, St-Martens-Latem Belgium) using the Dice correlation coefficient. The dendrogram illustrating the clonal relatedness of isolates was plotted using the Unweighted Pairgroup Method with Arithmetic Averages (UPGMA) with a position tolerance value of 1%. Clonality scores of 85% and above were accepted as clonal isolates23,24.
Infection control measures before the outbreak
Education regarding cleaning, hand hygiene, disinfection, basic microbiology, and infection control was delivered as a part of the introduction program to all healthcare staff right after recruitment. Healthcare staff was monitored and assessed every month regularly for their compliance with infection control measures. Feedback on hand hygiene was delivered monthly, based on the monitoring results generated by anonymous observers. The hand hygiene score was calculated, and the goal was set to be greater than 90%. Education on appropriate hand hygiene was given by the infection control team after receiving feedback. The importance of aseptic techniques during aspiration was also emphasized. The surfaces of aspiration jars were wiped using an alcohol-based disinfectant. Right after the initiation of the pandemic, double gloves were in use. Cleaning solutions used for surfaces and appliances were peracetic acid solution (0.2%) and chloride solution (0.1%).
Infection control measures during the outbreak
Acinetobacter baumannii infection elimination program was created by the infection control team regarding infection prevention and control (IPC) measures25. This program consisted of extended measures on appropriate personal protective equipment (PPE) use, environmental screening, hand hygiene, isolation precautions, cleaning, and disinfection with the participation of nurses, doctors, and janitorial staff. Infection control measures before the A. baumannii outbreak and additional measures during the outbreak were compared in Table 1.
Monitoring across all shifts was conducted daily under the supervision of the lead nurse of the infection control team. COVID-19 and A. baumannii infection patients were followed on contact, droplet, and airborne precautions. Non-disposable equipment was cleaned using 1000 ppm (0.1%) chlorine solution daily and for each visit. The use of double gloves was terminated as it has led to the continuation of using the inner glove after disposing of the outer glove. Aspiration jars were disinfected by soaking in 0.1% hypochlorite solution instead of wiping the surface of the jars with a chlorine solution (0.1%). Disposal of PPE was done after each examination and bedside visit. All equipment was disinfected before the removal from the room. Healthcare workers have received training on appropriate donning and doffing of PPE (gowns, gloves, goggles, or face shields). Hand hygiene monitoring has been implemented weekly instead of monthly.
The environmental screening was performed by infection control nurses in the patient rooms where A. baumannii was isolated after cleaning. Screening consisted of door handles, bedsides, IV pumps, aspiration jars, drawers, the surface of alcohol-based disinfectant bottles, monitors, ventilators, and stethoscopes per each room. Each room was blindly cleaned twice by two janitorial staff after each patient. Extended cleaning and disinfection training was given to all janitorial staff as practice (chlorine solution preparation, cleaning procedures, use of a single cleaning cloth). Clonality surveillance of A. baumannii isolates was done using the PFGE method.
Eventually, COVID-19 and A. baumannii infection patients were mainly isolated in rooms 1–8 which is a separate area within the ICU. The patients were taken to the other corridor with room numbers 9–16 only when rooms 1–8 were fully occupied.
Statistical analysis
Descriptive statistics of continuous variables were performed with medians and interquartile ranges. For categorical values frequencies were compared. The chi-square test and Mann–Whitney U Test were used to analyze the relationship between binary variables and continuous variables respectively. All the analyses were conducted via Stata 17.0 (StataCorp LLC, Texas USA) and a p-value less than 0.05 was considered statistically significant.
Ethics approval
All methods were carried out in accordance with relevant guidelines and regulations after receiving ethics approval. This study was approved by the Koç University Institutional Review Board (No: 2022.192.IRB1.069). Informed Consent was obtained from all patients included in the study.