Neurogenesis is disrupted in human hippocampal progenitor cells upon exposure to serum samples from hospitalized COVID-19 patients with neurological symptoms | Molecular Psychiatry
Treatment with serum samples from COVID-19 patients with delirium decreased cell proliferation, neurogenesis, and increased apoptosis, when compared with serum from non-delirium patients
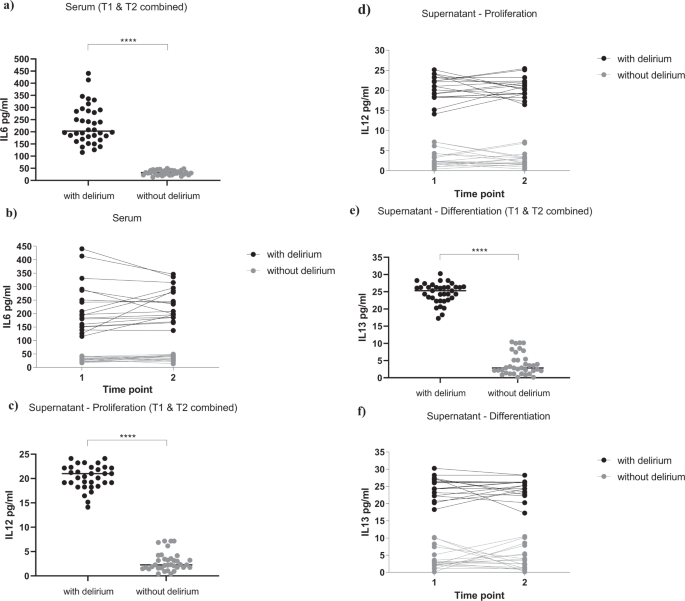
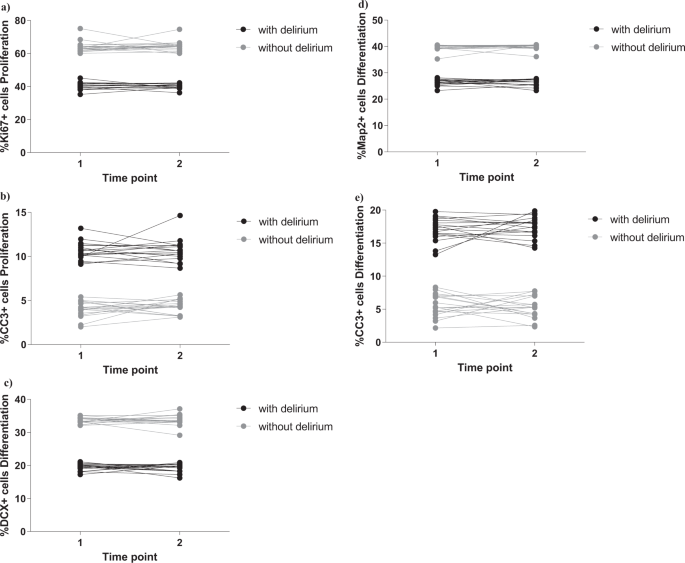
As in our established protocol [28], we exposed cells to 2 days treatment during proliferation, with 1% serum sample from each COVID-19 patient with or without delirium, collected both at time of hospital admission (Time point 1) and during admission (Time point 2). We measured markers of cell proliferation (Ki67), apoptosis (CC3) and stemness (Sox2, Nestin) (Fig. 1a). Interestingly, we found that treatment of cells with serum samples from delirium patients decreased cell proliferation (group effect: p < 0.0001; time effect: p = 0.6, Fig. 2a and Supplementary Fig. 1i) and increased apoptosis (group effect: p < 0.0001; time effect: p = 0.5, Fig. 2b and Supplementary Fig. 1j), with no differences between cell treated with serum samples collected at Time point 1 or Time point 2.
a, b Cell treatment with serum samples from delirium patients decreased proliferation (KI67 + cells) and increased apoptosis (CC3 + cells), when compared with serum from non-delirium patients. There were no differences across Time point 1 and 2. c–e Treatment with serum samples from delirium patients decreased neurogenesis (DCX + and Map2+cells) and increased apoptosis (CC3 + cells), when compared with serum from non-delirium patients. Again, there were no differences across Time point 1 and 2. Two-way mixed ANOVA was performed.
We did not observe any difference in the marker of stemness (Sox2 or Nestin), when comparing the two groups (delirium vs non-delirium) and the two time points (Supplementary Fig. 2a, b).
In the differentiation experiments, after the 2 days of treatment during proliferation, we left cells to differentiate for additional 4 days, again in presence of 1% serum. We measured markers of neurogenesis (DCX, Map2), astrogliogenesis (S100β) and apoptosis (CC3) (Fig. 1b). We found that treatment of cells with serum samples from delirium patients decreased neurogenesis (DCX: group effect: p < 0.0001; time effect: p = 0.9, Fig. 2c and Supplementary Fig. 1k; Map2: group effect: p < 0.0001; time effect: p = 0.9, Fig. 2d and Supplementary Fig. 1l) and increased apoptosis (group effect: p < 0.0001; time effect: p = 0.9, Fig. 2e and Supplementary Fig. 1m), again with no differences between the two time points.
Also, we did not observe any differences in the marker of astrogliogenesis (S100β), when comparing the two groups (delirium vs non-delirium) and the two time points (Supplementary Fig. 2c).
Higher concentration of IL6 in serum samples from COVID-19 patients with delirium induces cells to produce IL12 and IL13, respectively during the proliferation and differentiation stage
In order to investigate the molecular mechanisms through which cell treatment with serum samples from delirium patients detrimentally affected cell proliferation, neurogenesis and apoptosis, we first measured levels of candidate cytokines (IL1β, IL2, IL4, IL6, IL8, IL10, IL12, IL13, TNF-α, IFN-γ) known to be modulated by the SARS-CoV-2 virus [11,12,13], in serum samples of both patients with delirium and without delirium.
Interestingly, we found a significantly higher concentration of IL6 in serum samples of patients with delirium (229.9 ± 79.1 pg/ml, Fig. 3a, b), when compared with serum samples from patients without delirium (32.5 ± 9.5 pg/ml, Fig. 3a, b), again with no differences between serum samples collected at Time point 1 or Time point 2 (group effect: p < 0.0001; time effect: p = 0.4, Fig. 3b). None of the other cytokines were differentially expressed between the two groups or the two time points (Supplementary Fig. 3a–i).
a, b Serum samples from delirium patients showed increased levels of IL6, when compared with non-delirium patients, both at Time point 1 and 2. c, d During proliferation, exposure of cells with serum samples from delirium patients (collected both at Time point 1 and 2) induced the production of IL12 in the supernatant, when compared with treatment with serum samples from non-delirium patients. e, f During differentiation, exposure of cells with serum samples from delirium patients (collected both at Time point 1 and 2) induced the production of IL13 in the supernatant, when compared with treatment with serum samples from non-delirium patients. Mann–Whitney U-test and two-way mixed ANOVA were performed. Data are shown as mean; ****p < 0.0001 comparisons as indicated.
We then measured the same panel of cytokines in supernatant of cells exposed to treatment with serum samples from patients with or without delirium (collected both at Time point 1 and 2). In particular, cell supernatant was collected after 1 day of serum treatment during proliferation, and 1 day of serum treatment during differentiation (Fig. 1a, b).
Results showed significantly higher levels of IL12 during the proliferation, and IL13 during the differentiation, in the supernatant of cells treated with serum from delirium patients vs non-delirium patients (IL12: 20.6 ± 2.7 pg/ml vs 2.8 ± 2 pg/ml, Fig. 3c, d; IL13: 24.6 ± 3 pg/ml vs 3.9 ± 3.1 pg/ml, Fig. 3e, f), with no differences between supernatant of cells treated with serum samples collected at Time point 1 or Time point 2 (IL12: group effect: p < 0.0001; time effect: p = 0.8, Fig. 3d; IL13: group effect: p < 0.0001; time effect: p = 0.9, Fig. 3f).
Of note, concentrations of the same cytokines (IL12 and IL13) in the original serum samples were much lower, and did not differ between delirium and non-delirium patients (IL12: 2.1 ± 1 pg/ml vs 2.05 ± 1 pg/ml, Supplementary Fig. 3f; IL13: 2.7 ± 0.7 pg/ml vs 2.8 ± 1 pg/ml, Supplementary Fig. 3g), indicating that these differences in the supernatant levels are due to new production of these cytokines by the cells.
Finally, none of the other cytokines were differentially expressed in the supernatant of cells treated with serum samples from delirium or non-delirium patients, or from Time point 1 or Time point 2 (Supplementary Figure 3j-zz).
During proliferation, treatment with an antibody against IL6 prevents the detrimental effect of serum from COVID-19 patients with delirium on both cell proliferation and apoptosis, and decreases IL12 production
In order to confirm that the detrimental effect of treatment with serum from delirium patients on cell proliferation and apoptosis was due to the higher levels of IL6 in the serum samples of the same patients, we exposed cells to treatment with serum from COVID-19 patients together with an antibody against IL6 (Fig. 1c).
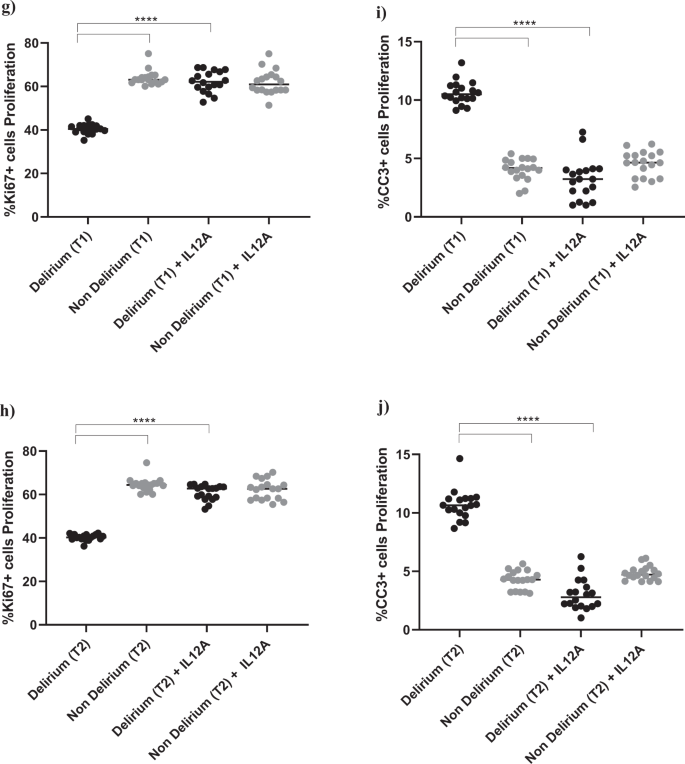
Interestingly, treatment with IL6 antibody prevented the decrease in proliferation (Ki67) and increase in apoptosis (CC3) caused by treatment with serum from delirium patients, when compared with treatment with serum from non-delirium patients (Time point 1, delirium vs delirium + IL6 antibody; for Ki67, 40.2% vs 61.3%, p < 0.0001; for CC3, 10.2% vs. 3.4%, p < 0.0001), with no differences between cells treated with serum samples collected at Time point 2 (Fig. 4a–d).
a–d Cell treatment with IL6A (0.1 μg/ml) prevented the decrease in proliferation (Ki67+cells) and increase in apoptosis (CC3 + cells) previously observed upon treatment with serum samples from patients with delirium. This is confirmed across treatment with serum samples collected at Time point 1 and 2. e, f Treatment with IL6A also prevented production of IL12 in cell supernatant. g–j Cell treatment with IL12A (0.3 μg/ml) prevented the decrease in proliferation (Ki67+cells) and increase in apoptosis (CC3 + cells) previously observed upon treatment with serum samples from patients with delirium. Again, this is confirmed across treatment with serum samples collected at Time point 1 and 2. Two-way mixed ANOVA with Bonferroni’s post hoc test was performed. Data are shown as mean; ****p < 0.0001 comparisons as indicated.
Moreover, treatment of cells with serum samples from delirium patients and IL6 antibody prevented the production of IL12 in cell supernatant (Time point 1, delirium vs delirium + IL6 antibody; IL12: 20.6 ± 2.7 pg/ml vs 4.1 ± 2.1 pg/ml, p < 0.0001,), again with no differences between Time point 1 and Time point 2 (Figs. 1c, 4e, f).
This finding seems to suggest that, during proliferation, the detrimental effect on cell proliferation and apoptosis caused by IL6, which is produced in higher concentrations in serum from delirium patients, may be mediated by the production of IL12 in the brain environment. Indeed, treatment of cells with an antibody against IL12 prevented the decrease in cell proliferation (Ki67) and increase in apoptosis (CC3) caused by treatment with serum from delirium patients (Time point 1, delirium vs delirium + IL12 antibody; for Ki67, 40.2% vs 62.2%, p < 0.0001; for CC3, 10.2% vs 3.2%, p < 0.0001). Again, there were no differences between cells treated with serum samples collected at Time point 1 or Time point 2 (Figs. 1c, 4g–j).
During differentiation, treatment with an antibody against IL6 prevents the detrimental effect of serum from COVID-19 patients with delirium on both neurogenesis and apoptosis, and decreases IL13 production
In order to investigate whether the detrimental effect of treatment with serum from delirium patients on neurogenesis and apoptosis during differentiation was also mediated by a higher concentration of IL6 in the serum samples of the same patients, we exposed cells to treatment with serum and an antibody against IL6, first during proliferation, then during differentiation, and finally during both proliferation and differentiation (Fig. 1d, e).
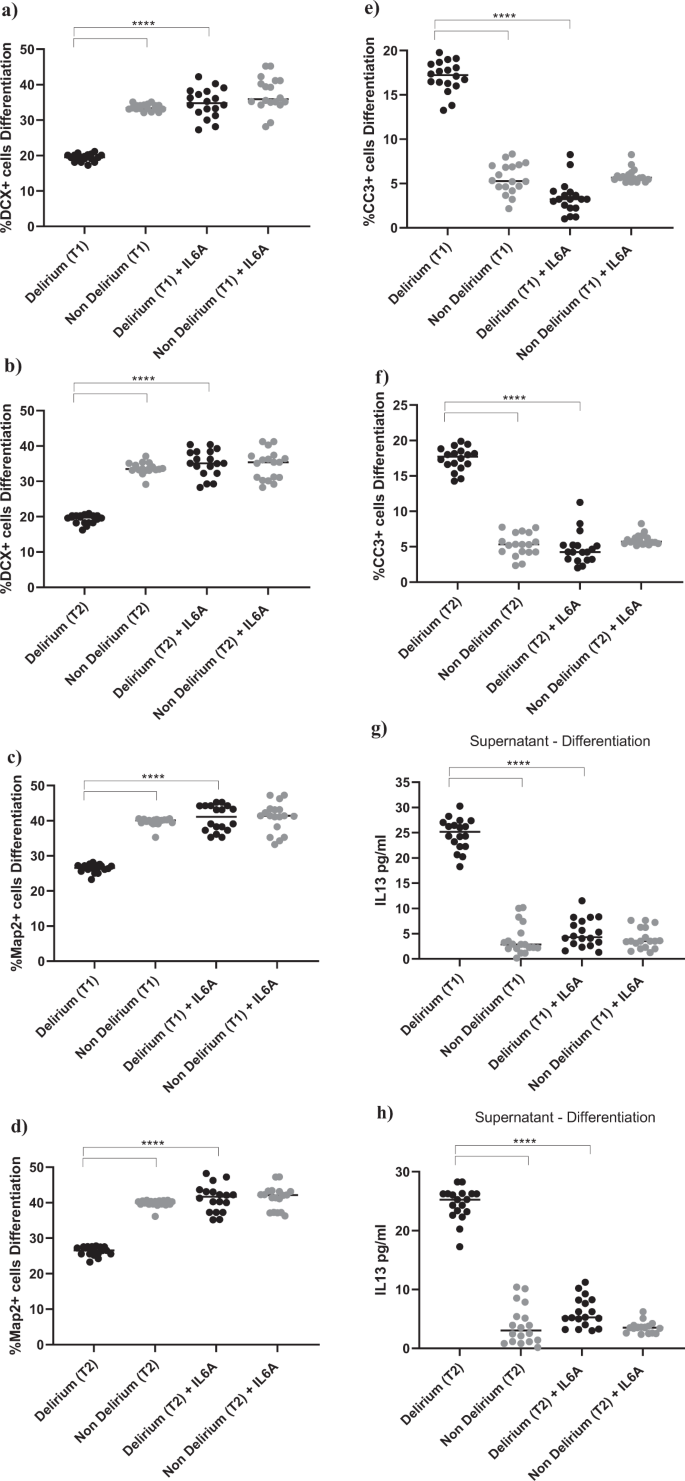
Exposing cells to IL6 antibody during the proliferation stage did not prevent the effect of serum on the aforementioned cellular outcomes (Supplementary Fig. 4a–f). However, treatment of cells with IL6 antibody during the differentiation stage prevented the reduction in neurogenesis (DCX, Map2) and increase in apoptosis (CC3) previously observed upon treatment with serum from delirium patients, when compared with serum from non-delirium patients (Time point 1, delirium vs delirium + IL6 antibody; for DCX, 19.1% vs 34.8%, p < 0.0001; for Map2, 25.1% vs 40.7%, p < 0.0001; for CC3, 17.3% vs 3.4%, p < 0.0001). Again, there were no differences between cells Time point 1 and Time point 2 (Fig. 5a–f). Results were confirmed when exposing cells to IL6 antibody during both the proliferation and differentiation stage (Supplementary Fig. 4g–l).
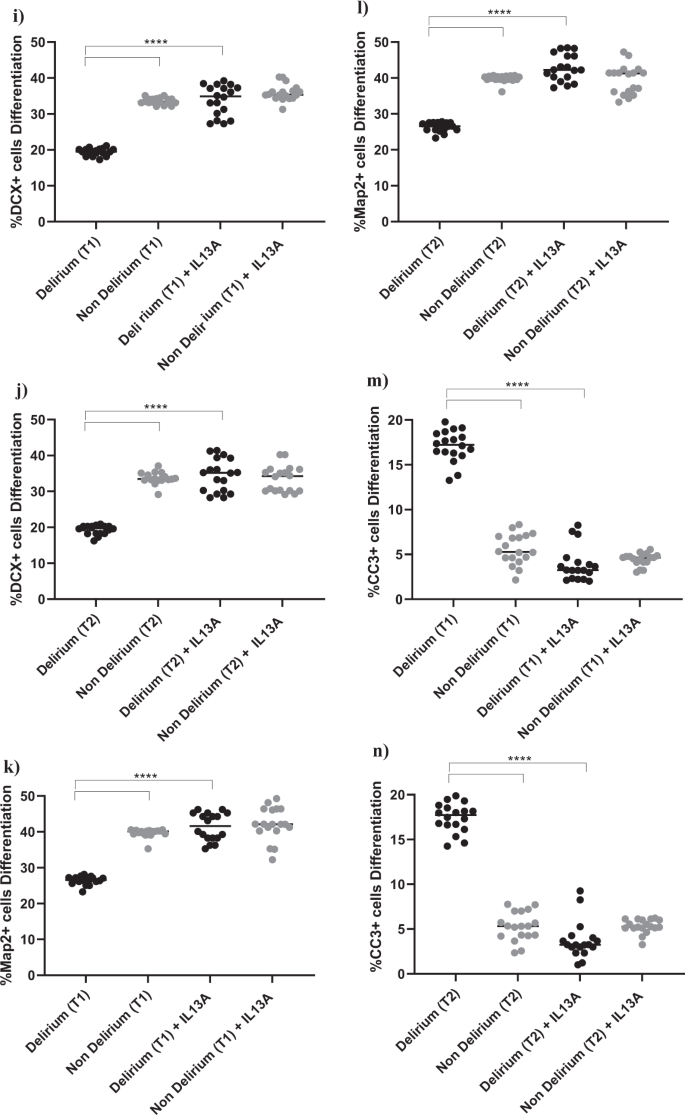
a–f Cell treatment with IL6A (0.1 μg/ml) prevented the decrease in neurogenesis (DCX + and Map2+cells) and increase in apoptosis (CC3 + cells) previously observed upon treatment with serum samples from patients with delirium. This is confirmed across treatment with serum samples collected at Time point 1 and 2. g, h Treatment with IL6A also prevented production of IL13 in cell supernatant. i–n Cell treatment with IL13A (0.1ug/ml) prevented the decrease in neurogenesis (DCX + and Map2+cells) and increase in apoptosis (CC3 + cells) previously observed upon treatment with serum samples from patients with delirium. Again, this is confirmed across treatment with serum samples collected at Time point 1 and 2. Two-way mixed ANOVA with Bonferroni’s post hoc test was performed. Data are shown as mean; ****p < 0.0001 comparisons as indicated.
Moreover, similar to the effects of IL6 antibody during the proliferation phase on the production of IL12, treatment of cells with serum samples from delirium patients and IL6 antibody during the differentiation stage prevented the production of IL13 in cell supernatant, (Time point 1, delirium vs delirium + IL6 antibody; IL13: 24.6 ± 3 pg/ml vs 5.1 ± 2.7 pg/ml, p < 0.0001,), again with no differences between Time point 1 and Time point 2 (Figs. 1e, 5g, h).
In this case, finding seems to suggest that, during differentiation, the detrimental effect on neurogenesis and apoptosis caused by the high IL6 concentrations in serum from delirium patients is mediated by production of IL13. Indeed, treatment of cells with an antibody against IL13 prevented the decrease in neurogenesis (DCX, Map2) and increase in apoptosis (CC3) caused by treatment with serum from delirium patients (Time point 1, delirium vs delirium + IL13 antibody; for DCX, 19.1% vs 33.8%, p < 0.0001; for Map2, 25.1% vs 41.4%, p < 0.0001; for CC3, 17.3% vs 3.8%, p < 0.0001). Again, there were no differences between Time point 1 and Time point 2 (Figs. 1c, 5i–n).
Treatment with recombinant IL12 or IL13, at concentrations previously found in supernatant of cells exposed to serum from patients with delirium, decreases cell proliferation, neurogenesis and increase apoptosis, whereas co-treatment with the JAK inhibitors, baricitinib, ruxolitinib and tofacitinib, prevents these detrimental effects
Having identified the downstream production of IL12 and IL13 in supernatant of cells exposed to serum from patients with delirium as the mechanism responsible for the reduced cell proliferation and neurogenesis and increased apoptosis, we decided to test whether (1) these same effects could be replicated by treating cells directly with IL12 and IL13 at the same concentrations measured in the supernatant following treatment with serum; and (2) if treatment with effective and commonly administered therapeutic compounds for patients with acute COVID-19, the JAK inhibitors baricitinib, ruxolitinib and tofacitinib (all 1 nM), could prevent these detrimental effects by IL12 and IL13.
For this purpose, we treated cells directly with recombinant IL12 and IL13, used at concentrations previously found in supernatant of cells exposed to serum from patients with delirium (IL12 delirium: 20 pg/ml and IL13 delirium: 25 pg/ml) or without delirium (IL12 without delirium: 3 pg/ml, IL13 without delirium: 4 pg/ml, Fig. 1f, g), with or without the JAK inhibitors.
As hypothesised, during proliferation, treatment with IL12 (20 pg/ml) was able to cause a decrease in cell proliferation (Ki67) and increase in apoptosis (CC3), when compared with IL12 (3 pg/ml) (for Ki67, 42.8% vs 71.6%, p < 0.0001; for CC3, 11.5% vs 5.6%, p < 0.0001, Supplementary Fig. 5a, b). This is similar to what previously shown upon exposure of cells to serum from patients. Interestingly, co-treatment with baricitinib, ruxolitinib or tofacitinib were able to prevent these detrimental effects (IL12 20 pg/ml vs IL12 3 pg/ml + vehicle/baricitinab/ruxolitinab/tofacitinab; for Ki67, 42.8% vs 68.4%, p < 0.001, vs 69%, p < 0.001, vs 71.4%, p < 0.001; for CC3, 11.5% vs 5.7%, p < 0.0001, vs 5%, p < 0.0001, vs 5.4%, p < 0.0001, Supplementary Fig. 5a, b).
Similarly, during differentiation, treatment with IL13 (25 pg/ml) was able to cause a decrease in neurogenesis (DCX and Map2) and increase in apoptosis (CC3), when compared with IL13 (4 pg/ml) (for DCX, 20.8% vs 31%, p < 0.0001; for Map2, 27.2% vs 41%, p < 0.0001; for CC3, 15.8% vs 7.6%, p < 0.0001, Supplementary Fig. 5c–e). This is, again, similar to what previously shown upon exposure of cells to serum from patients. Again, co-treatment with baricitinib, ruxolitinib or tofacitinib were able to prevent these detrimental effects (IL13 25 pg/ml vs IL13 4 pg/ml + vehicle/baricitinab/ruxolitinab/tofacitinab; for DCX, 20.8% vs 30.4%, p < 0.001, vs 29%, p < 0.001, vs 30%, p < 0.001; for Map2, 27.2% vs 39.1%, p < 0.001, vs 39.7%, p < 0.001, vs 39.7%, p < 0.001; for CC3, 15.8% vs 7.4%, p < 0.0001, vs 8.3%, p < 0.0001, vs 8.1%, p < 0.0001, Supplementary Fig. 5c–e).