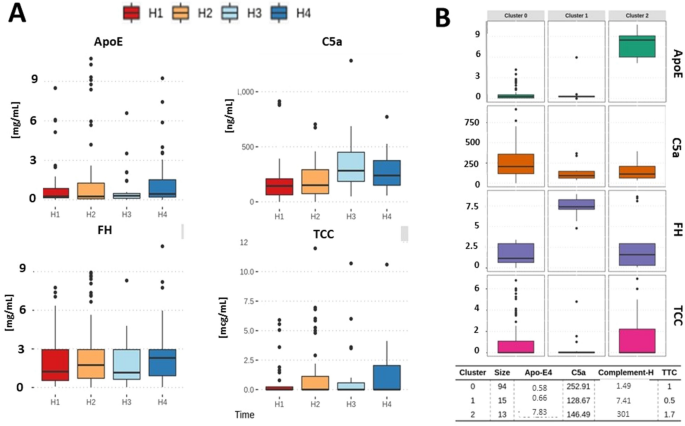
A disturbed balance between blood complement protective factors (FH, ApoE) and common pathway effectors (C5a, TCC) in acute COVID-19 and during convalesce | Scientific Reports

Rendeiro, A. F. et al. Profiling of immune dysfunction in COVID-19 patients allows early prediction of disease progression. Life Sci. Alliance 4. https://doi.org/10.26508/lsa.202000955 (2021).
Chevrier, S. et al. A distinct innate immune signature marks progression from mild to severe COVID-19. Cell Rep. Med. 2, 100166. https://doi.org/10.1016/j.xcrm.2020.100166 (2021).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062. https://doi.org/10.1016/s0140-6736(20)30566-3 (2020).
Reyes Gil, M. et al. Correlation of coagulation parameters with clinical outcomes during the coronavirus-19 surge in New York: Observational cohort. Front. Physiol. 12, 618929. https://doi.org/10.3389/fphys.2021.618929 (2021).
Posch, W. et al. C5aR inhibition of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2-infected primary human airway epithelia. J. Allergy Clin. Immunol. 147, 2083-2097.e2086. https://doi.org/10.1016/j.jaci.2021.03.038 (2021).
Holter, J. C. et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. U.S.A. 117, 25018–25025. https://doi.org/10.1073/pnas.2010540117 (2020).
Yan, B. et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci. Immunol. 6. https://doi.org/10.1126/sciimmunol.abg0833 (2021).
Rawish, E., Sauter, M., Sauter, R., Nording, H. & Langer, H. F. Complement, inflammation and thrombosis. Br. J. Pharmacol. 178, 2892–2904. https://doi.org/10.1111/bph.15476 (2021).
Skerka, C. et al. Factor H-related protein 1: A complement regulatory protein and guardian of necrotic-type surfaces. Br. J. Pharmacol. 178, 2823–2831. https://doi.org/10.1111/bph.15290 (2021).
Parente, R., Clark, S. J., Inforzato, A. & Day, A. J. Complement factor H in host defense and immune evasion. Cell. Mol. Life Sci. 74, 1605–1624. https://doi.org/10.1007/s00018-016-2418-4 (2017).
Page, E. M. & Ariëns, R. A. S. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb. Res. 200, 1–8. https://doi.org/10.1016/j.thromres.2021.01.005 (2021).
Zhang, Y. et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2007575 (2020).
Noris, M., Benigni, A. & Remuzzi, G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 98, 314–322. https://doi.org/10.1016/j.kint.2020.05.013 (2020).
Kang, Y.-H., Tan, L. A., Carroll, M. V., Gentle, M. E. & Sim, R. B. Target pattern recognition by complement proteins of the classical and alternative pathways. Target Pattern Recognit. Innate Immun. 117–128 (2009).
Unnewehr, H. et al. Changes and regulation of the C5a receptor on neutrophils during septic shock in humans. J. Immunol. 190, 4215–4225. https://doi.org/10.4049/jimmunol.1200534 (2013).
Gaca, J. G. et al. Effect of an anti-C5a monoclonal antibody indicates a prominent role for anaphylatoxin in pulmonary xenograft dysfunction. Transplantation 81, 1686–1694. https://doi.org/10.1097/01.tp.0000226063.36325.02 (2006).
Xie, C. B., Jane-Wit, D. & Pober, J. S. Complement membrane attack complex: New roles, mechanisms of action, and therapeutic targets. Am. J. Pathol. 190, 1138–1150. https://doi.org/10.1016/j.ajpath.2020.02.006 (2020).
Wu, C. P. et al. A meta-analysis. Front. Med. (Lausanne) 8(603558), 2021. https://doi.org/10.3389/fmed.2021.603558 (2019).
Hotchkiss, R. S. et al. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2, 16045. https://doi.org/10.1038/nrdp.2016.45 (2016).
Wang, L. et al. Pentraxin 3 recruits complement factor H to protect against oxidative stress-induced complement and inflammasome overactivation. J. Pathol. 240, 495–506. https://doi.org/10.1002/path.4811 (2016).
Martin, M. & Blom, A. M. Complement in removal of the dead—balancing inflammation. Immunol. Rev. 274, 218–232. https://doi.org/10.1111/imr.12462 (2016).
Lipcsey, M. et al. The outcome of critically Ill COVID-19 patients is linked to thromboinflammation dominated by the kallikrein/kinin system. Front. Immunol. 12, 627579. https://doi.org/10.3389/fimmu.2021.627579 (2021).
Sjöberg, A., Onnerfjord, P., Mörgelin, M., Heinegård, D. & Blom, A. M. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 280, 32301–32308. https://doi.org/10.1074/jbc.M504828200 (2005).
Trouw, L. A. et al. C4b-binding protein and factor H compensate for the loss of membrane-bound complement inhibitors to protect apoptotic cells against excessive complement attack. J. Biol. Chem. 282, 28540–28548. https://doi.org/10.1074/jbc.M704354200 (2007).
Leffler, J. et al. Annexin-II, DNA, and histones serve as factor H ligands on the surface of apoptotic cells. J. Biol. Chem. 285, 3766–3776. https://doi.org/10.1074/jbc.M109.045427 (2010).
Alic, L. et al. A genome-wide association study identifies key modulators of complement factor H binding to malondialdehyde-epitopes. Proc. Natl. Acad. Sci. USA. 117, 9942–9951. https://doi.org/10.1073/pnas.1913970117 (2020).
Kárpáti, É. et al. Interaction of the factor H family proteins FHR-1 and FHR-5 with DNA and dead cells: implications for the regulation of complement activation and opsonization. Front. Immunol. 11, 1297. https://doi.org/10.3389/fimmu.2020.01297 (2020).
Sjöberg, A. P. et al. The factor H variant associated with age-related macular degeneration (His-384) and the non-disease-associated form bind differentially to C-reactive protein, fibromodulin, DNA, and necrotic cells. J. Biol. Chem. 282, 10894–10900. https://doi.org/10.1074/jbc.M610256200 (2007).
Kang, Y. H., Urban, B. C., Sim, R. B. & Kishore, U. Human complement Factor H modulates C1q-mediated phagocytosis of apoptotic cells. Immunobiology 217, 455–464. https://doi.org/10.1016/j.imbio.2011.10.008 (2012).
Yalcin Kehribar, D. et al. The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomark. Biochem. Indicators Exposure Response Suscept. Chem. 26, 114–118. https://doi.org/10.1080/1354750x.2020.1861099 (2021).
Andersson, U. & Tracey, K. J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162. https://doi.org/10.1146/annurev-immunol-030409-101323 (2011).
Huang, W., Tang, Y. & Li, L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 51, 119–126. https://doi.org/10.1016/j.cyto.2010.02.021 (2010).
Olivar, R. et al. The complement inhibitor factor H generates an anti-inflammatory and tolerogenic state in monocyte-derived dendritic cells. J. Immunol. 196, 4274–4290. https://doi.org/10.4049/jimmunol.1500455 (2016).
Smolag, K. I. et al. Complement inhibitor factor H expressed by breast cancer cells differentiates CD14(+) human monocytes into immunosuppressive macrophages. Oncoimmunology 9, 1731135. https://doi.org/10.1080/2162402x.2020.1731135 (2020).
Pilling, D., Galvis-Carvajal, E., Karhadkar, T. R., Cox, N. & Gomer, R. H. Monocyte differentiation and macrophage priming are regulated differentially by pentraxins and their ligands. BMC Immunol. 18, 30. https://doi.org/10.1186/s12865-017-0214-z (2017).
Laine, M. et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J. Immunol. 178, 3831–3836. https://doi.org/10.4049/jimmunol.178.6.3831 (2007).
Stravalaci, M. et al. Control of complement activation by the long pentraxin PTX3: Implications in age-related macular degeneration. Front. Pharmacol. 11, 591908. https://doi.org/10.3389/fphar.2020.591908 (2020).
Vogt, L. M. et al. Apolipoprotein E triggers complement activation in joint synovial fluid of rheumatoid arthritis patients by binding C1q. J. Immunol. 204, 2779–2790. https://doi.org/10.4049/jimmunol.1900372 (2020).
Soto, I. et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 13, e1002279. https://doi.org/10.1371/journal.pbio.1002279 (2015).
Klos, K. et al. APOE/C1/C4/C2 hepatic control region polymorphism influences plasma apoE and LDL cholesterol levels. Hum. Mol. Genet. 17, 2039–2046. https://doi.org/10.1093/hmg/ddn101 (2008).
Nissilä, E. et al. Complement factor H and Apolipoprotein E participate in regulation of inflammation in THP-1 macrophages. Front. Immunol. 9, 2701. https://doi.org/10.3389/fimmu.2018.02701 (2018).
Garner, B., Mellor, H. R., Butters, T. D., Dwek, R. A. & Platt, F. M. Modulation of THP-1 macrophage and cholesterol-loaded foam cell apolipoprotein E levels by glycosphingolipids. Biochem. Biophys. Res. Commun. 290, 1361–1367. https://doi.org/10.1006/bbrc.2002.6356 (2002).
Pogue, A. I. et al. Characterization of an NF-kappaB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J. Inorg. Biochem. 103, 1591–1595. https://doi.org/10.1016/j.jinorgbio.2009.05.012 (2009).
Ma, L. et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. bioRxiv. https://doi.org/10.1101/2021.02.22.432177 (2021).
Brasen, C. L. et al. Daily monitoring of viral load measured as SARS-CoV-2 antigen and RNA in blood, IL-6, CRP and complement C3d predicts outcome in patients hospitalized with COVID-19. Clin. Chem. Lab. Med. https://doi.org/10.1515/cclm-2021-0694 (2021).
Gratz, J. et al. Risk of clinically relevant venous thromboembolism in critically ill patients with COVID-19: A systematic review and meta-analysis. Front. Med. (Lausanne) 8, 647917. https://doi.org/10.3389/fmed.2021.647917 (2021).
Nannoni, S., de Groot, R., Bell, S. & Markus, H. S. Stroke in COVID-19: A systematic review and meta-analysis. Int. J. Stroke 16, 137–149. https://doi.org/10.1177/1747493020972922 (2021).
McGonagle, D., Bridgewood, C., Ramanan, A. V., Meaney, J. F. M. & Watad, A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol 3, e224–e233. https://doi.org/10.1016/s2665-9913(20)30420-3 (2021).
Vitiello, A., La Porta, R., D’Aiuto, V. & Ferrara, F. Pharmacological approach for the reduction of inflammatory and prothrombotic hyperactive state in COVID-19 positive patients by acting on complement cascade. Hum. Immunol. https://doi.org/10.1016/j.humimm.2021.01.007 (2021).
Li, Y. et al. Complement inhibition ameliorates blast-induced acute lung injury in rats: Potential role of complement in intracellular HMGB1-mediated inflammation. PLoS ONE 13, e0202594. https://doi.org/10.1371/journal.pone.0202594 (2018).
Ye, Z. et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: A systematic review and meta-analysis. CMAJ 192, E756–E767. https://doi.org/10.1503/cmaj.200645 (2020).
Prescott, H. C. & Rice, T. W. Corticosteroids in COVID-19 ARDS: Evidence and hope during the pandemic. JAMA 324, 1292–1295. https://doi.org/10.1001/jama.2020.16747 (2020).
Kolilekas, L. et al. Can steroids reverse the severe COVID-19 induced “cytokine storm”?. J. Med. Virol. 92, 2866–2869. https://doi.org/10.1002/jmv.26165 (2020).
Roback, J. D. & Guarner, J. Convalescent plasma to treat COVID-19: Possibilities and challenges. JAMA 323, 1561–1562. https://doi.org/10.1001/jama.2020.4940 (2020).
Aviani, J. K., Halim, D., Soeroto, A. Y., Achmad, T. H. & Djuwantono, T. C. (COVID-19) treatment: A systematic review and meta-analysis based on recent studies and previous respiratory pandemics. Rev. Med. Virol. https://doi.org/10.1002/rmv.2225 (2019).
Ng, K. K., Ng, M. K., Zhyvotovska, A., Singh, S. & Shevde, K. Acute respiratory failure secondary to COVID-19 viral pneumonia managed with hydroxychloroquine/azithromycin treatment. Cureus 12, e8268. https://doi.org/10.7759/cureus.8268 (2020).
Singh, A. K., Singh, A., Singh, R. & Misra, A. Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metabol. Syndrome 14, 641–648. https://doi.org/10.1016/j.dsx.2020.05.018 (2020).
Beigel, J. H. et al. Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 383, 1813–1826. https://doi.org/10.1056/NEJMoa2007764 (2020).
Acosta-Ampudia, Y. et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 118, 102598. https://doi.org/10.1016/j.jaut.2021.102598 (2021).
Barie, P. S., Hydo, L. J. & Fischer, E. Comparison of APACHE II and III scoring systems for mortality prediction in critical surgical illness. Arch. Surg. 130, 77–82 (1995).
Buntinx, F. et al. Evaluation of Charlson’s comorbidity index in elderly living in nursing homes. J. Clin. Epidemiol. 55, 1144–1147 (2002).
Peres Bota, D., Melot, C., Lopes Ferreira, F., Nguyen Ba, V. & Vincent, J. L. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med. 28, 1619–1624 (2002).
Cuenca, A. G. et al. The Glue Grant experience: Characterizing the post injury genomic response. Eur. J. Trauma Emerg. Surg. 37, 549–558. https://doi.org/10.1007/s00068-011-0148-8 (2011).
Venkataraman, R. & Kellum, J. A. Defining acute renal failure: The RIFLE criteria. J. Intensive Care Med. 22, 187–193. https://doi.org/10.1177/0885066607299510 (2007).
Cavalcanti, A. B. et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N. Engl. J. Med. 383, 2041–2052. https://doi.org/10.1056/NEJMoa2019014 (2020).
Remy, K. E. et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 5, 1–15. https://doi.org/10.1172/jci.insight.140329 (2020).
Coopersmith, C. M. et al. Surviving sepsis campaign: Research priorities for sepsis and septic shock. Crit. Care Med. 46, 1334–1356. https://doi.org/10.1097/ccm.0000000000003225 (2018).
Coopersmith, C. M. et al. The surviving sepsis campaign: Research priorities for coronavirus disease 2019 in critical illness. Crit. Care Med. 49, 598–622. https://doi.org/10.1097/ccm.0000000000004895 (2021).
Torres Rives, B. et al. Serum immunoglobulin levels, complement components 3 and 4, HLA-B27 allele and spondyloarthropathy in patients with non-infectious anterior uveites. Reumatol. Clin. https://doi.org/10.1016/j.reuma.2020.07.007 (2020).
Wang, R., Xiao, H., Guo, R., Li, Y. & Shen, B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg. Microbes Infect. 4, e28. https://doi.org/10.1038/emi.2015.28 (2015).
Loftus, T. J. et al. Overlapping but disparate inflammatory and immunosuppressive responses to SARS-CoV-2 and bacterial sepsis: An immunological time course analysis. Front. Immunol. 12, 792448. https://doi.org/10.3389/fimmu.2021.792448 (2021).
Tomazini, B. M. et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 324, 1307–1316. https://doi.org/10.1001/jama.2020.17021 (2020).
Villar, J. et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 8, 267–276. https://doi.org/10.1016/s2213-2600(19)30417-5 (2020).
Sugimoto, M. A., Sousa, L. P., Pinho, V., Perretti, M. & Teixeira, M. M. Resolution of inflammation: What controls its onset?. Front. Immunol. 7, 160. https://doi.org/10.3389/fimmu.2016.00160 (2016).
Sallenave, J. M. & Guillot, L. Innate immune signaling and proteolytic pathways in the resolution or exacerbation of SARS-CoV-2 in Covid-19: Key therapeutic targets?. Front Immunol. 11, 1229. https://doi.org/10.3389/fimmu.2020.01229 (2020).