Fluid restriction management in the treatment of COVID-19: a single-center observational study | Scientific Reports

Patients
COVID-19 patients admitted to our hospital between July 2020 and October 2021 were included in the analysis. The study period corresponds to the second to fifth waves in Japan. We mainly treated patients with severe respiratory failure (requiring ventilatory support) or those with the potential for severe respiratory failure and patients who were COVID-19 positive and require emergency care (surgery, endovascular therapy, etc.) [“COVID-19 positive emergency patients”]. We often collaborate with the hospitals in charge of mild and moderate illnesses to accommodate patients transferred to the hospital. In some cases, patients with severe respiratory failure are brought in directly by ambulance, and difficult-to-accommodate cases are accepted during nights and holidays to maintain the emergency medical system.
The institutional policy of COVID-19 treatment
In principle, the COVID-19 treatment policy was based on the Coronavirus Disease 2019 (COVID-19) Treatment Guidelines17. The Japanese guidelines are publicly available and have been updated. The essential drug therapy was remdesivir and dexamethasone (6 mg/day)6. The steroid (methylprednisolone) dose was increased to 2 mg/kg when the patient was judged to be critically ill based on CT, oxygenation, and rate of deterioration. Patients without bacterial infection or immunosuppression were treated with tocilizumab or baricitinib, in addition to remdesivir and steroids. Until the third wave, HFNC was only used after extubation. After the fourth wave (May 2021), HFNC was actively introduced before mechanical ventilation9. If excessive inspiratory effort and tachypnea are not resolved even after HFNC is started, tracheal intubation and mechanical ventilation should be started as soon as possible. In the patients with severe obesity18, young age, no delirium, and severe chronic obstructive pulmonary disease, HFNC with high oxygen concentration could be continued with awake prone patients11 to avoid artificial respiration. In mechanically ventilated patients, esophageal pressure was monitored to titrate the positive end-expiratory pressure (PEEP)19. When the CT showed a dorsal or unilateral predominant shadow distribution, positional therapy (prone or lateral position) was used. Although indications of the ECMO are very different, such as refractory hypoxia or hypercapnia, VV-ECMO was introduced.
Patients on ventilators or ECMO were administered continuous enteral nutrition starting at 20 mL/h with peptide-based formula (Peptamen AF®, Nestle HealthCare Nutrition, Inc.) and increased the injection rate daily. The target calorie dose (approximately 1500 kcal/day) is usually achieved on day 4. In addition to continuous intravenous insulin, oral hypoglycemic agents (biguanides, dipeptidyl peptidase-4 inhibitors, sodium-glucose cotransporter-2 inhibitors, and thiazolidinediones) have been used to control glucose intolerance due to steroid use and frequent complications of diabetes mellitus. To control excessive inspiratory effort, patients with severe COVID-19 often require high doses of opioids. Naldemedine20 was used in addition to magnesium oxide and sodium picosulfate to promote intestinal peristalsis. Since most severe COVID-19 cases were hemodynamically stable, we attempted fluid restriction management. We restricted the injection fluid and used diuretics or continuous renal replacement therapy if needed in acute kidney injury or chronic kidney disease patients.
Measurement
The patients’ background (age, sex, length, weight, blood test on admission) and admission route (direct ambulance transport, A; transfer from the outside hospital, T) were recorded. Severity was defined as oxygen therapy (non-intubated, Group 1 [G1]; ventilated, Group 2 [G2]; ECMO, Group 3 [G3]). We collected data on ventilator duration, ECMO duration, drug treatment (total steroid dose, tocilizumab, baricitinib), sequelae, blood transfusion requirement, in-hospital mortality, hospital length of stay (LOS), and waiting period (days between extubation and transfer or discharge).
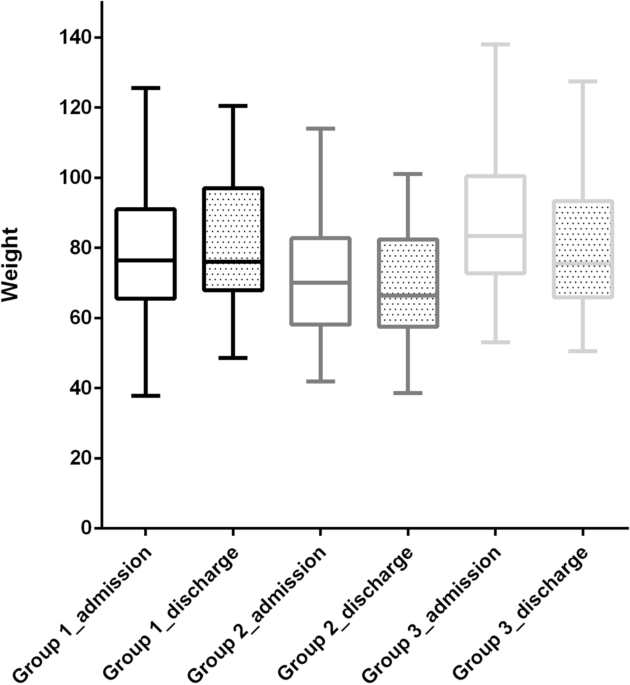
Patient characteristics, clinical course, and weight loss during hospitalization were compared according to illness severity. In the severe group (G2 + G3). We then compared the characteristics, clinical course, and admission route. We examined the hospital LOS and waiting period for each severity of illness among the patients who were discharged alive.
Statistical analyses
Scale data are expressed as median (25th–75th percentile) and categorical data as number (percentage). Group comparisons were made using the Jonckheere–Terpstra test or Mann–Whitney U test for scale data and Chi-square test for categorical data, with P < 0.05 considered significant. The primary outcome was hospital LOS, and the secondary outcomes were in-hospital mortality and the waiting period.
Ethics approval
This study was approved at the institutional review board at Chiba Emergency Medical Centre (Oct 11, 2021). This research has been performed in accordance with the Declaration of Helsinki and relevant guidelines/regulations. Informed consent was waived by the Ethics Committee.